Ready to implement NGS? Interested in NGS data analysis?
The Holotype HLA Assay is licensed from The Children’s Hospital of Philadelphia (CHOP) and provides amplification and library preparation reagents for comprehensive gene coverage up to 11 HLA loci.
The Software, Omixon HLA Twin, features two independent algorithms for double validation by data analysis to deliver the most accurate, high-resolution genotyping available, with no reflexive testing required.
Competitive Advantage - Gene Coverage up to 11 HLA Loci
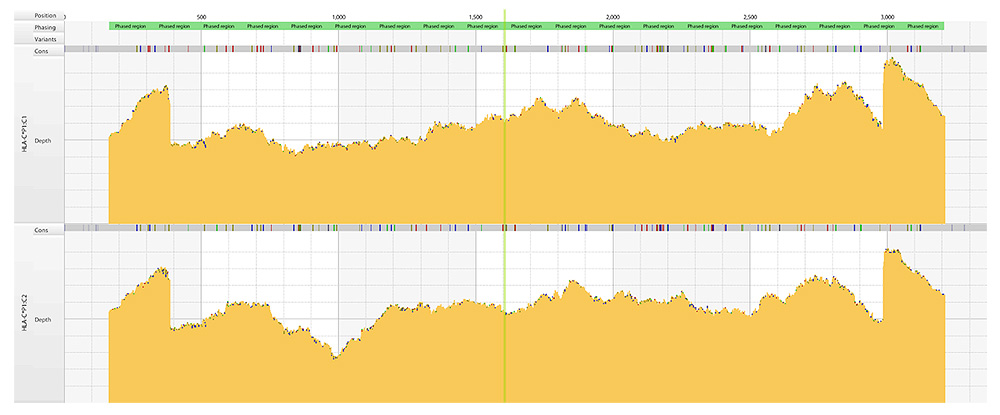
Holotype HLA provides deep and even coverage of the whole region, with balance between alleles at the same locus, between the loci within a sample and also between samples – up to 96 samples per Miseq run at 11 loci, up to 192 samples at 7 loci and 288 samples at 5 loci - enabling better phasing, reduced ambiguity, and optimal utilization of sequence output.
Coverage plots
Deep and even coverage of two HLA-C alleles assembled simultaneously by the Consensus Genotyping (CG) algorithm in HLA Twin and displayed in the Gene Browser.
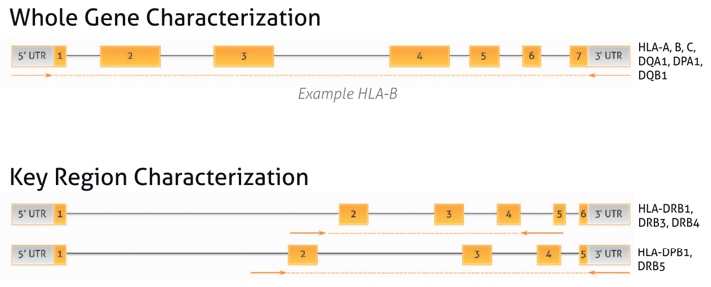
Amplification Strategy
The location of the primers provides comprehensive coverage of up to 11 HLA loci with no clinically relevant ambiguities remaining.
All Omixon products are available as RUO and certain configurations of Holotype HLA are available as CE-IVD in Europe where marked.