Written by Dr. Attila Berces, Chairman at Omixon. Share the original article on LinkedIn here…
“We try to remember that medicine is for the patient. We try never to forget that medicine is for the people. It is not for the profits. The profits follow, and if we have remembered that, they have never failed to appear. The better we have remembered it, the larger they have been.” — George W. Merck, December 1, 1950
Pharmaceutical companies pursuing personalized or precision medicine have struggled to find the right business models to turn patient benefits into profits. A crucial point of precision medicine is to reduce the treatable patient population to responders by a diagnostic test. As long as the healthcare system pays for the treatment and not for the results, there is a strong financial disincentive to voluntarily introduce such a test.
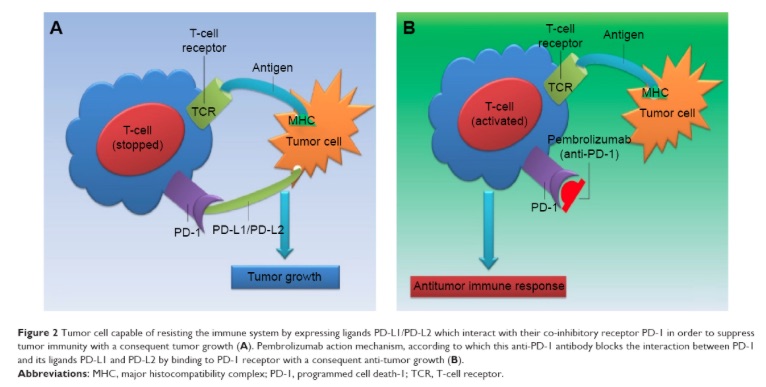
A prime example for such a financial disincentive are the two competing cancer immunotherapies: Opdivo from Bristol-Myers Squibb and Keytruda from Merck. Both antibodies disrupt cellular PD-1 receptor/PD-L1 ligand interaction in order to enhance the immune system’s ability to fight cancer. Experts and clinicians consider the therapeutic performance of Opdivo and Keytruda as virtually identical to one another in similar patient populations. In contrast, 2015 financial performance of Opdivo and Keytruda were remarkably different. Opdivo, had $942 million in sales versus Keytruda’s $605 million, and Opdivo was growing twice as fast.
Although some of the differences can be attributed to differences in marketing approaches, but the most significant difference is that Keytruda requires a diagnostic test, while Opdivo does not. The diagnostic test measures the extracellular expression level of PD-L1 protein, which is the natural ligand for PD-1 receptor and the interaction of which both Opdivo and Keytruda are designed to inhibit. While studies confirmed that higher levels of PD-L1 can be linked to a higher therapeutic response, some patients with low level of PD-L1 also respond to the therapy. BMS has created a competitive advantage from its more liberal label for Opdivo. In a BMS television ad campaign the ad’s voiceover intones “No biomarker testing is required with Opdivo, though physicians may choose to do so.”
In August BMS reported that Opdivo failed to show patient benefit in previously untreated patients with non-small cell lung cancer. This news has been particularly shocking in the light of Merck’s announcement in June of a successful clinical trial in the same stage and indication. The news immediately wiped out $20B of the market capitalization of BMS and increased Merck’s market cap by about the same. How could the two similar, some even consider identical treatments have performed so differently? The answer can be found in Merck’s use of companion biomarker testing and is an attribute of the companies more precise assessment of different patient populations. Merck chose PD-L1 levels of at least 50% as a patient selection criterion for its clinical trial, while BMS chose a more liberal PD-L1 level of at least 5%. While this strategy worked well for BMS in the advanced stage of the disease, it failed as first line therapy.
Why did this difference not show up in previous clinical trials? Cancer therapies are first tested in patients with advanced stage of disease who have exhausted most conventional therapeutic options; typically heavily pre-treated, metastatic patients. The clinical benefit is the easiest to demonstrate in this setting. In contrast, to introduce a first-line therapy, the clinical trial must demonstrate benefit in comparison to current first-line standard of care. Both Opdivo and Keytruda earned breakthrough designation in the advanced stages of cancer, and significantly extended the lives of a fraction of patients who would otherwise have faced imminent death. Opdivo on the other hand has failed to show benefit over current first line therapy, which also works for a fraction of patients. In this first-line setting, selecting the right patient population for the clinical trial is more critical.
PD-L1 levels are only one of the necessary, but not sufficient criteria for checkpoint inhibitors to work. The cancer itself must be antigenic. The cancer DNA must include mutations, which produce peptide antigens that the immune system recognizes as foreign. Exogenous carcinogens such as sunlight or cigarette smoke introduce random mutations that may or may not be antigenic. The presence of the mutated peptide is also not sufficient. They must fit into the binding pocket of the human leukocyte antigens (HLA) of the patient and HLA must be expressed on the cell surface. This is the reason why checkpoint inhibitors only work for a small fraction of patients. Heavy smokers, on the other hand, have highly mutated cancers with many potential antigens to be recognized by the immune system and thus they respond better to the therapy.
As the level of competition increases for checkpoint inhibitors, we shall see more refined biomarkers introduced as companion or complementary diagnostics. These diagnostics will help to select the right patients who can truly benefit from checkpoint inhibitors.
The failure of Opdivo is a landslide victory for personalized medicine and shows that billions of dollars of value may depend on the use of the right diagnostic tests.
Reference: Image reproduced from G. Improta et al. “New developments in the management of advanced melanoma – role of pembrolizumab“